Our Science
Harnessing the regenerative power of the human body
Pioneering brain-targeting chemistry platform optimizes and enhances the delivery of small molecules to the CNS
-
Our Approach
-
Brain-Targeting Chemistry Platform
-
Publications
01
Our Approach
Our unique approach to CNS drug development combines three distinct elements:
-
Brain-Targeting Chemistry Platform
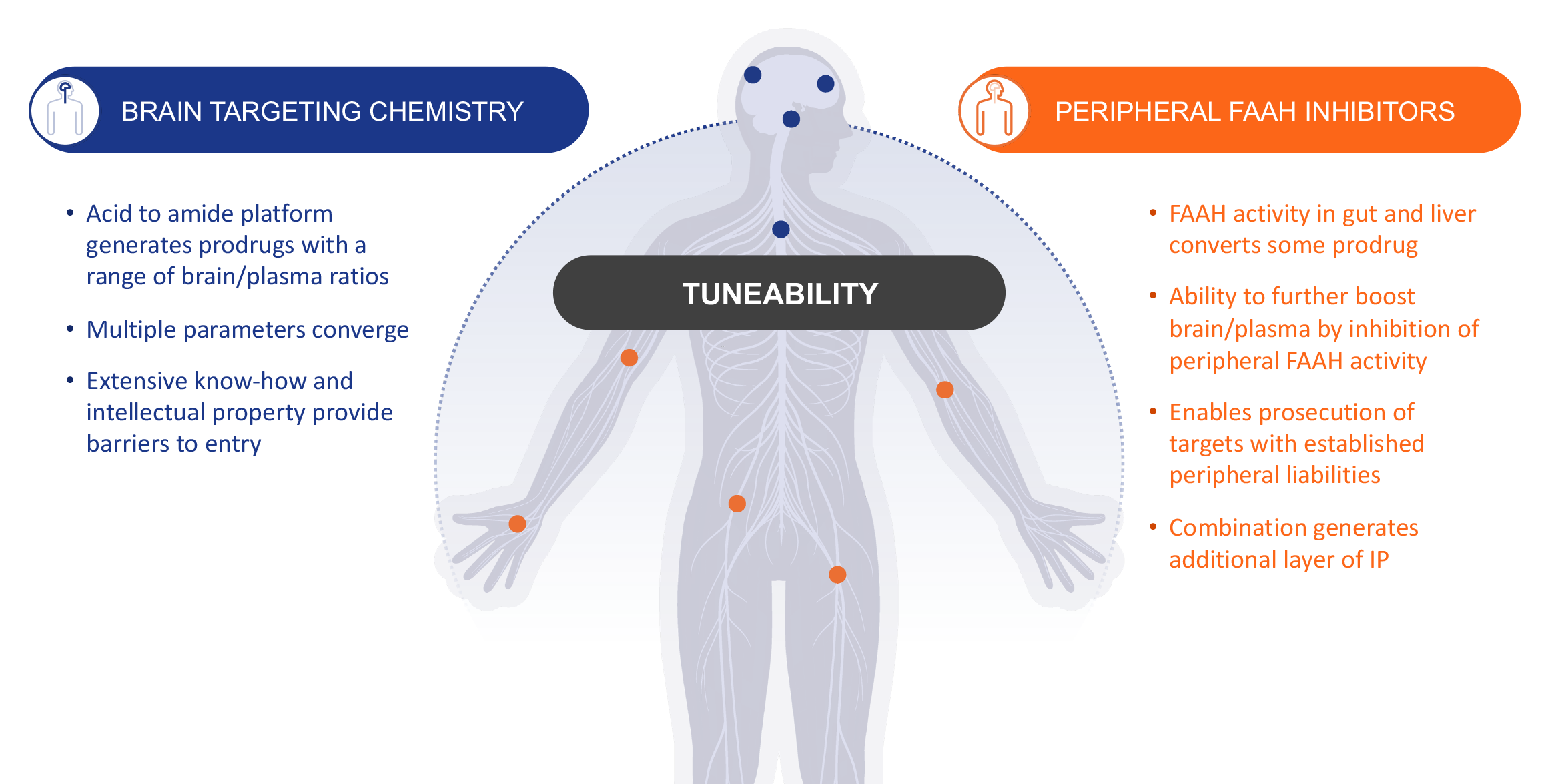
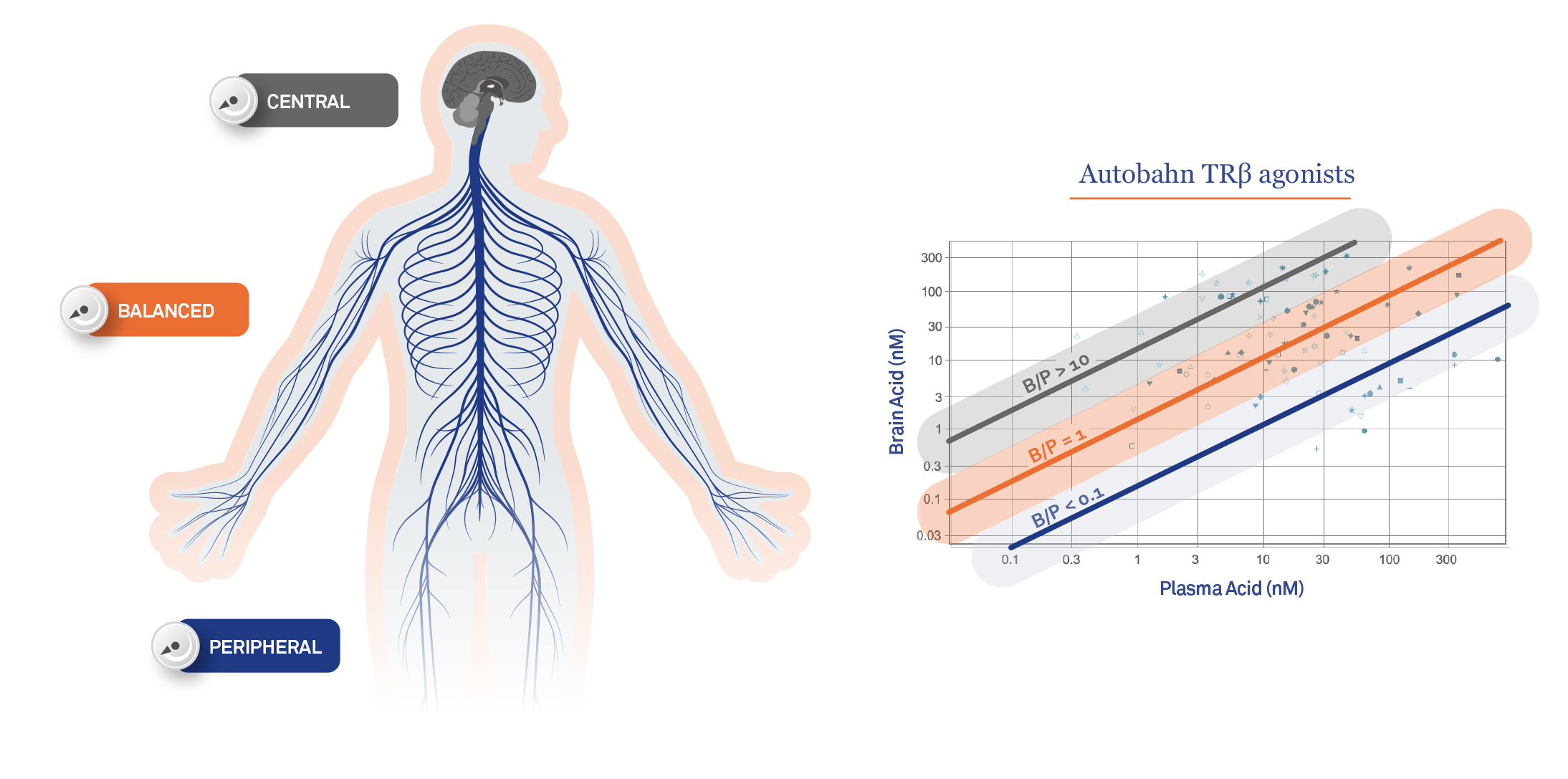
Our brain-targeting chemistry platform creates orally administered small molecule prodrugs with tunable ratios of central vs. peripheral exposure. This approach enables us to develop molecules with bespoke distribution profiles tailored to match disease pathophysiology.
-
Validated Human Biology
Our portfolio is anchored by high-confidence programs that leverage established clinical precedence, human genetics and biology, de-risking our pipeline and accelerating early development.
-
Biomarker-Driven Development
Our programs utilize biomarker-driven development strategies to demonstrate on-target / on-tissue activity and proof-of-mechanism early in the clinic.
Collectively, these elements enable us to de-risk and accelerate the development of our transformative small molecule therapies.
Brain-Targeting Chemistry Platform
02
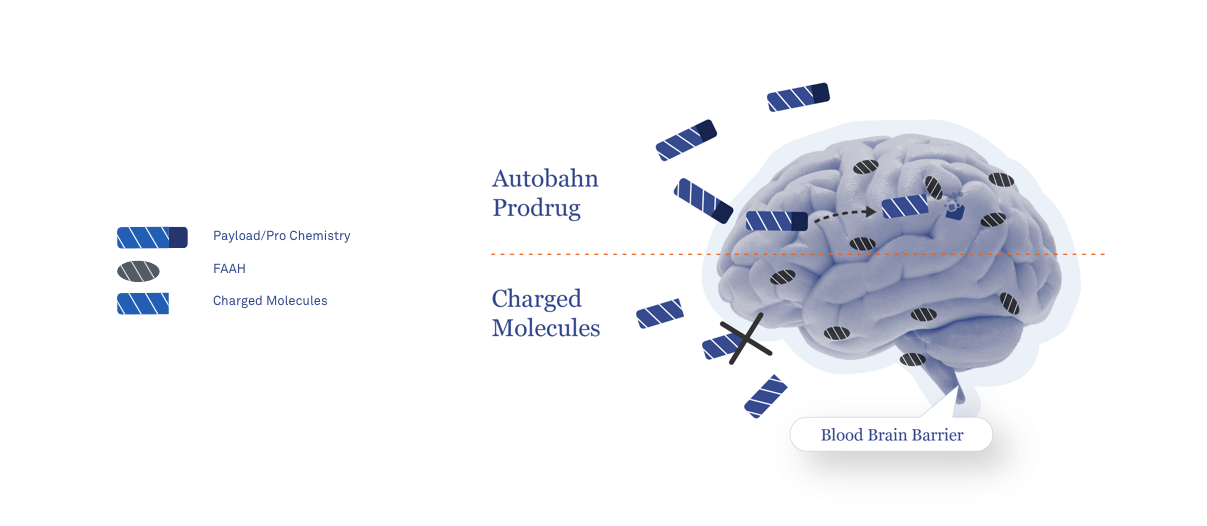
The blood-brain barrier is composed of tightly linked endothelial cells that limit the passage of pathogens and specific types of small and large molecules from the blood into the brain. This critical protective function also restricts the diffusion of therapeutics into the brain representing a major challenge to the development of new medicines for CNS diseases.
By combining the highly specialized enzymatic activity and brain-focused tissue distribution profile of fatty-acid amide hydrolase (FAAH), amplified with novel peripherally restricted FAAH inhibitors, our prodrug and peripheral restriction technology enables us to precisely tailor CNS and peripheral drug exposure.
Optimizing central versus peripheral exposure of active molecules enables us to match biodistribution with disease pathophysiology, providing Autobahn with a range of opportunities to leverage our platform to develop new therapeutics addressing unmet human health needs.
TEST
About Autobahn
About Us